Abstract
Introduction: The use of triple agent induction therapy before first line high-dose therapy (HDT) and autologous stem cell transplant (ASCT) in multiple myeloma is now considered standard of care. While thalidomide/bortezomib/dexamethasone (VTD) is the only induction regimen approved by EMA, both cyclophosphamide/bortezomib/dexamethasone (VCD) and lenalidomide/bortezomib/dexamethasone (RVD) are recommended in the most recent ESMO guidelines (Moreau et al., Annals of Oncology, 2017).
Prospective comparisons of VCD and RVD are lacking. Cross-study comparisons show that the combinations of proteasome inhibitors (PIs) and immunomodulatory drugs (IMIDs) have the highest response rates (Malankody Nat Rev Clin Onc 2015).
In recent years there has been a shift in choice of induction therapy from the VCD to the RVD regimen in our region. We have evaluated how this shift has affected depth of response 3 months after ASCT in all patients receiving first line HDT with ASCT in our region from January 1st, 2015, and how frequently our patients needed to change induction regimen, based on choice of first line therapy.
Methods: All patients receiving first line HDT with ASCT for multiple myeloma in our institution in the period from January 1st, 2015 to March 16th, 2018 were evaluated for the final analysis of response 3 months post-ASCT. The induction regimen was chosen at their local physician´s discretion. The patients received 3-5 cycles of induction therapy before leukapheresis, HDT (melphalan 200mg/m2) and ASCT. All patients received a follow-up consultation in our institution 3 months post-ASCT, where response was evaluated and recorded according to the IMWG 2016 response criteria. No patients had started maintenance therapy at the time of evaluation. Age, sex, date of ASCT, the presence of high-risk cytogenetics, ISS stage at diagnosis, choice of induction regimen and response 3 months post-ASCT was recorded by the primary investigator (Table 1). In case of change of regimen during induction treatment, the reason was recorded. Patients receiving either VCD or RVD as induction therapy, who did not change regimen during induction, were included in the final response analysis.
Results: 212 patients received HDT with ASCT in the period. 209 patients were evaluated 3 months after ASCT as of June 21st, 2018. 57.5% (122 patients) received VCD as first-line induction therapy, while 25.9% (55 patients) received RVD. 11.5% (14 patients) in the VCD group changed induction therapy vs 3.6% (2 patients) in the RVD group. Reasons for changing regimen were: not achieving at least a partial response (PR) (n=10), unacceptable toxicity (n=4), lack of documented reason (n=2). In the RVD group, 1 patient died from sepsis 2 weeks post-ASCT, and 1 patient refused to attend the post-ASCT evaluation. Therefore, 108 patients in the VCD group and 51 patients in the RVD group were included in the final response analysis.
29 patients could not confirm a complete response (CR) due to lack of bone marrow and/or serum immunofixation at response evaluation. For that reason, patients achieving CR, unconfirmed CR and very good partial response (VGPR) were compounded to a ≥VGPR response grade.
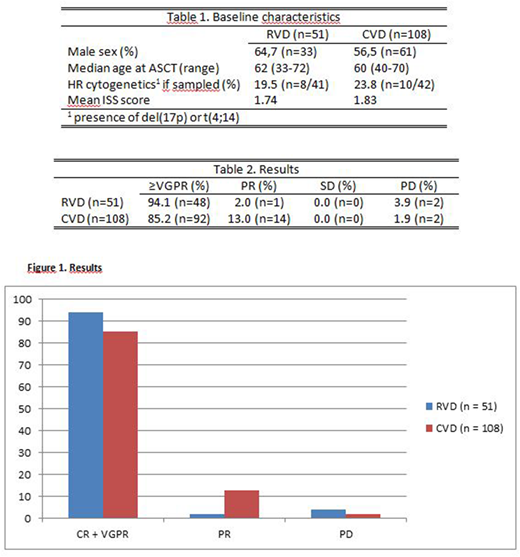
In the RVD group 94,1% (48 patients) achieved ≥VGPR vs 85,2% (92 patients) in the VCD group. Similar differences were also present for confirmed CR (25% vs 18%) and unconfirmed CR (44% vs 35%). In addition, 2% in the RVD group (1 patient) achieved PR, while 3.9% (2 patients) had disease progression (PD) 3 months after ASCT. In the VCD group, 13.0% (14 patients) achieved PR, while 1.8% (2 patients) had PD 3 months after ASCT (Table 2, Figure 1).
Discussion: RVD induction therapy before HDT with ASCT yielded higher rates of at least VGPR compared to VCD induction (85,2% s 94,1%). The choice of RVD as first line induction therapy necessitated fewer changes of induction regimen due to insufficient response or unacceptable toxicities compared to VCD induction (3,6% vs 11,5%). The differences were not statistically significant, possibly because of too few patients. Still, our results support the trend in our region of choosing RVD as first-line induction for transplant-eligible patients. These results should be confirmed in larger patient materials, and in prospective studies.
Updated data with approximately 35 additional patients, mainly receiving RVD induction, will be presented at the ASH annual meeting if our abstract is selected for presentation.
Moksnes:Shire: Consultancy. Schjesvold:Amgen: Consultancy, Honoraria, Research Funding; Celgene: Consultancy, Honoraria; Takeda: Consultancy, Honoraria; Bristol Myers Squibb: Consultancy; Bayer: Consultancy; Janssen: Consultancy, Honoraria, Research Funding; Oncopeptides: Consultancy; Abbvie: Honoraria; Adaptive: Consultancy; Novartis: Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal